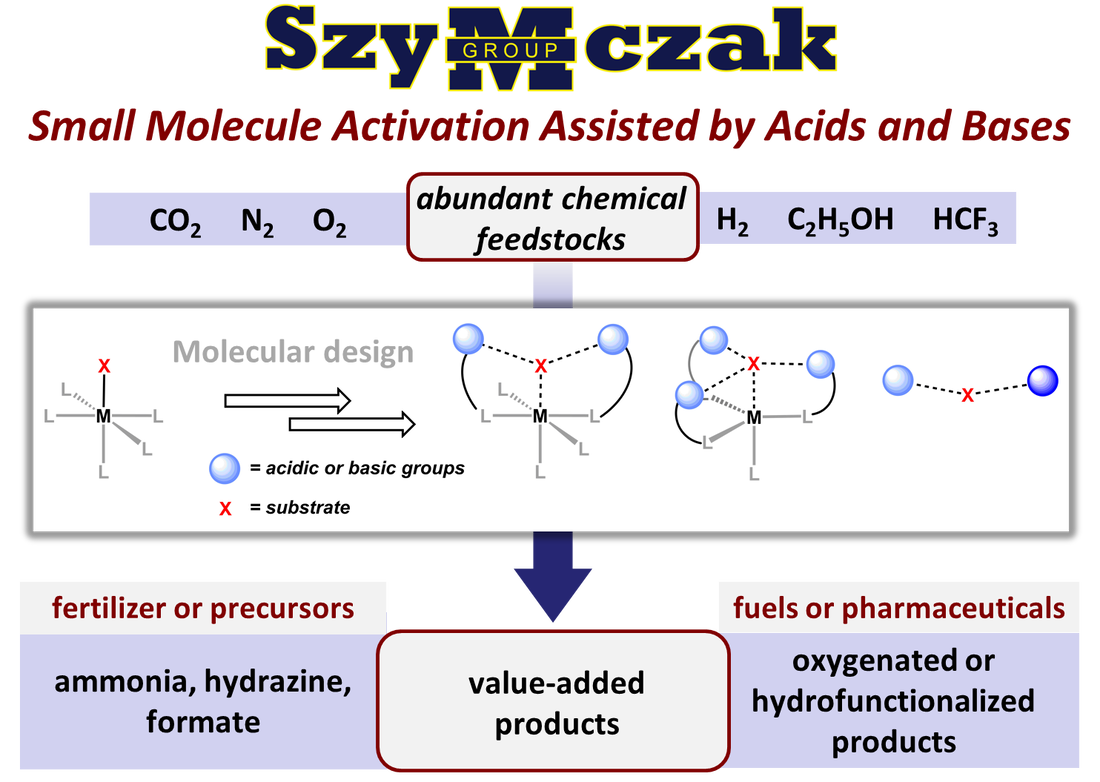
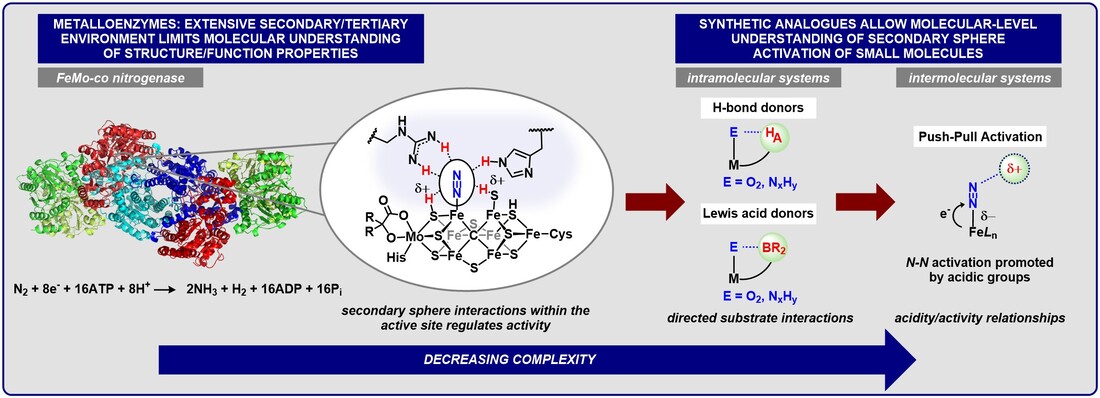
Bioinorganic Modeling: Decoding the role secondary sphere interactions play to sustain life
We are developing small molecule systems to directly investigate the molecular level details of how noncovalent interactions facilitate highly challenging enzymatic reactions. Key questions include how acidic groups can facilitate charge transfer and activation of the components of the air we breathe (N2, O2).
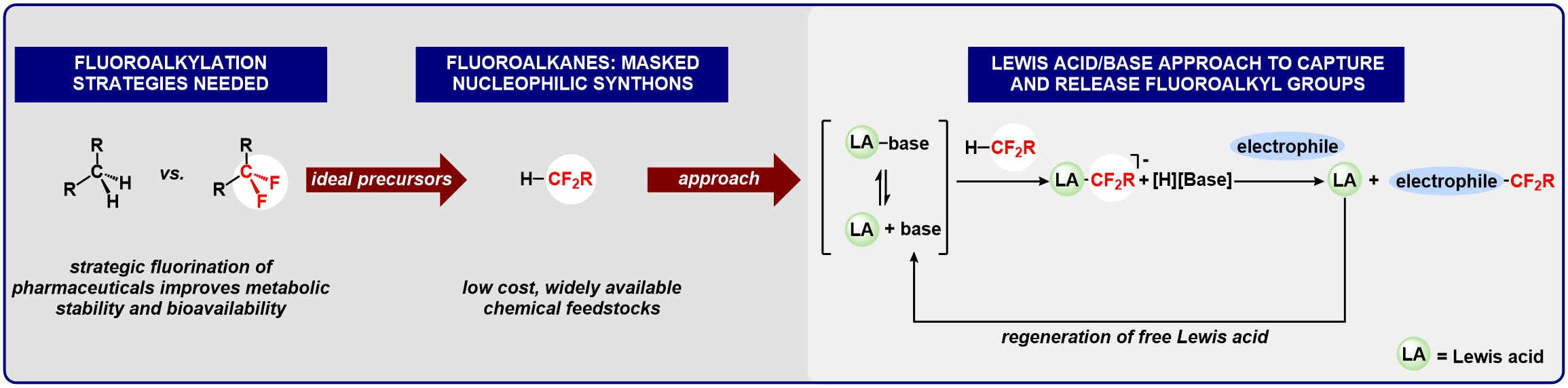
Fluoroalkylation: Repurposing fluoroalkanes using Lewis pairs
We are targeting an acid/base pair approach to enable widely available fluoralkanes to be used as direct chemical precursors to medicinally important organic compounds. In contrast to alkyl anions, fluoroalkyl anions are inherently unstable, and require the development of new synthetic strategies.
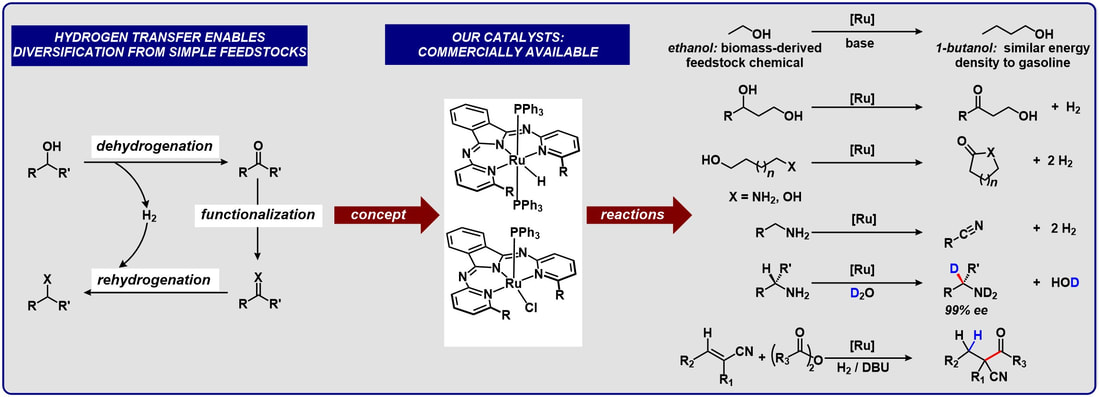
Hydrogen Transfer Catalysis: Upgrading low cost chemical feedstocks to value added fuels and platform chemicals
We are developing homogeneous catalysts that efficiently remove H2 from alcohols and amines and enable broader diversification from simple, widely available feedstock chemicals. In some cases, the dehydrogenated compounds can be functionalized in a cascade reaction sequence to rapidly increase molecular complexity.
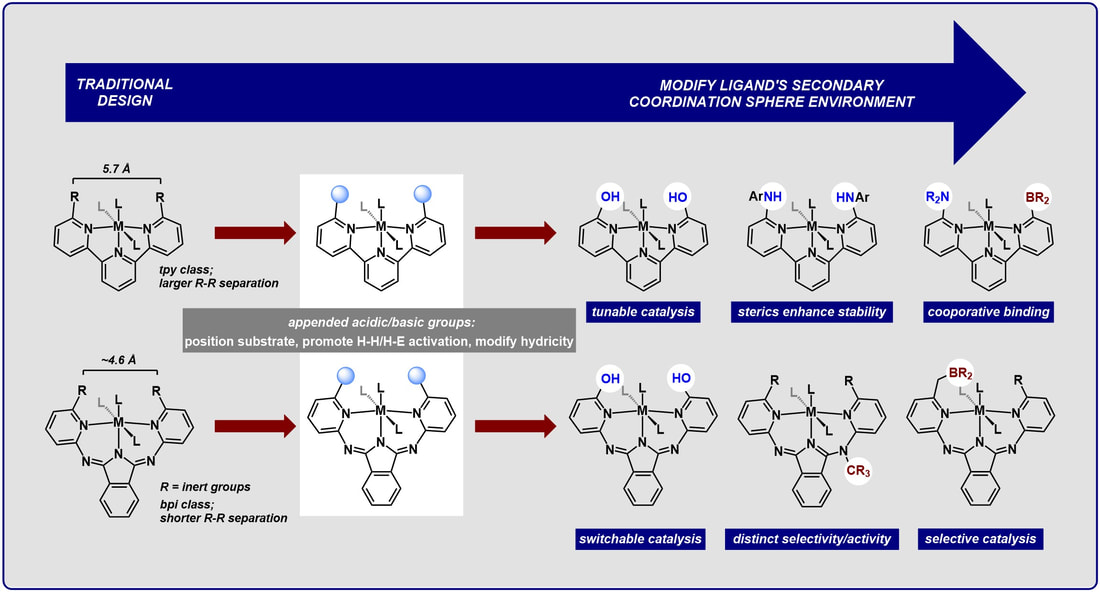
Second-Sphere Modifications for Catalyst Redesign: Uncovering cooperative activation and functionalization reactions
Our group takes inspiration from nature, where metalloenzymes use a network of positioned secondary sphere groups to achieve exquisite control over a given bond-breaking or forming reaction. To emulate these design principles, we are preparing new synthetic catalysts that use positioned acidic groups to direct substrate binding/activation to control a reaction’s chemo– and/or regioselectivity.