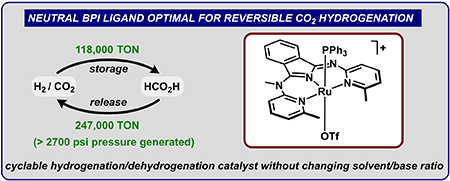
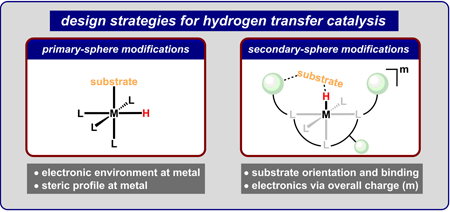
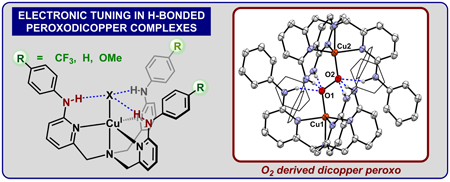
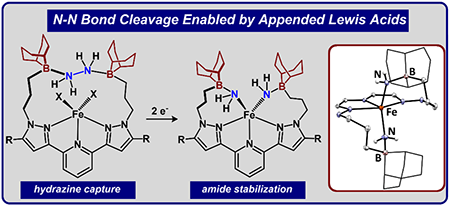
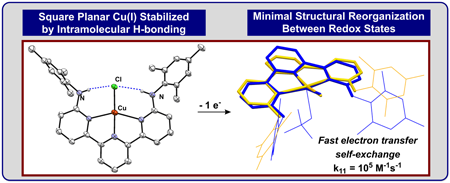
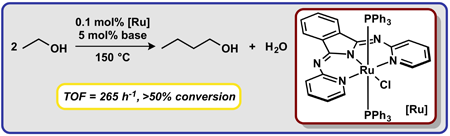
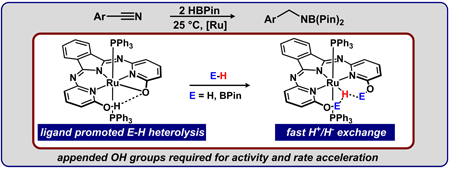
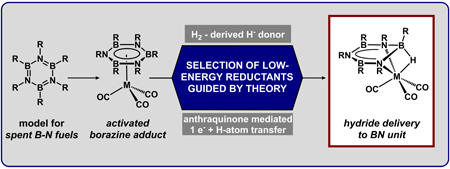
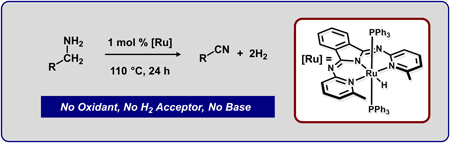
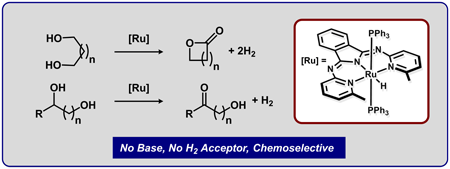
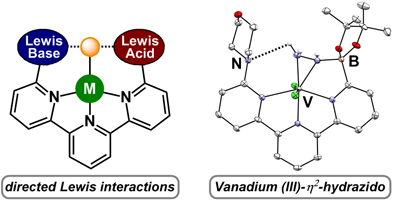
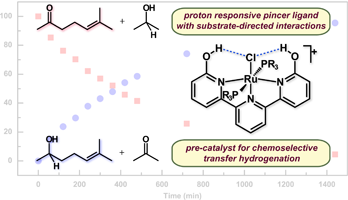
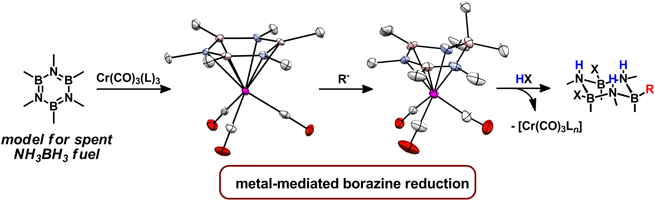
|
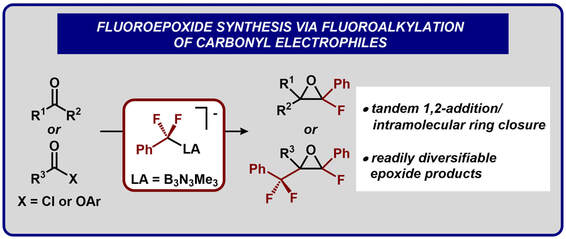
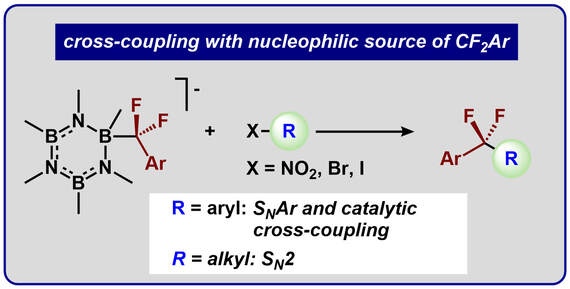
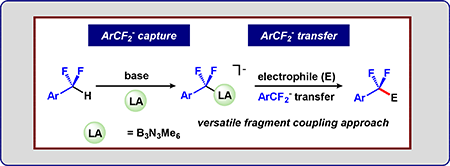
55.) Guo, S.; Sun, W.; Tucker, J. W.; Hesp, K. D.; Szymczak, N.K.* Preparation and Functionalization of Mono- and Polyfluoroepoxides via Fluoroalkylation of Carbonyl Electrophiles. Chem. Eur. J, 2022, doi/10.1002/chem.202203578 |
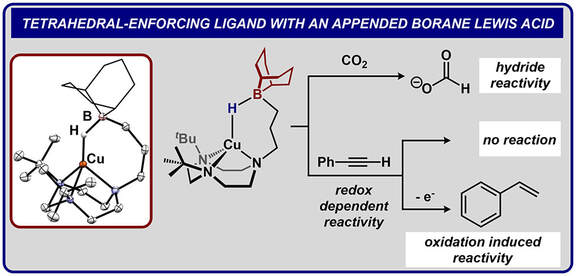
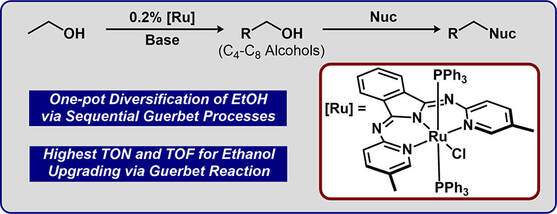
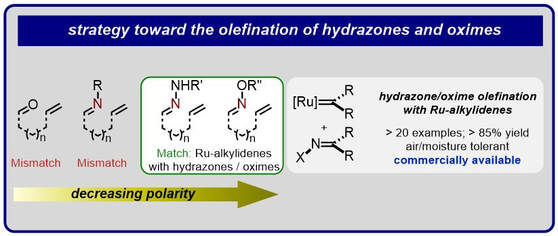
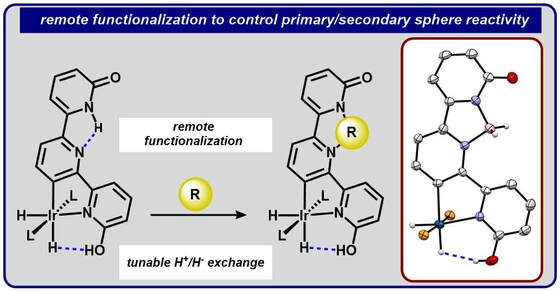
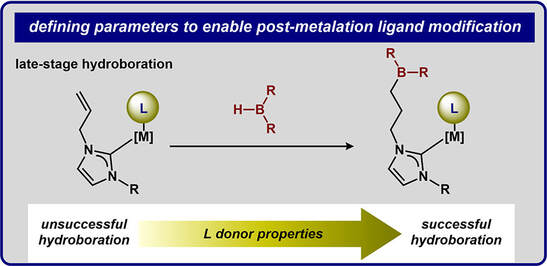
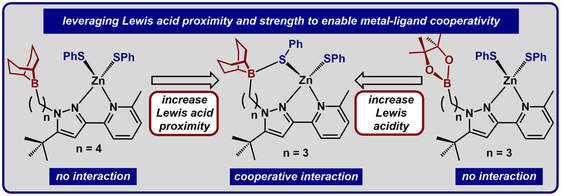
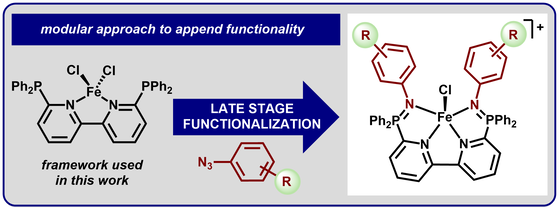
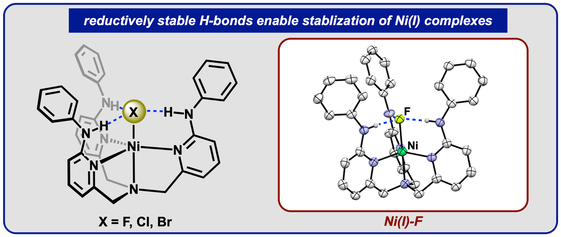
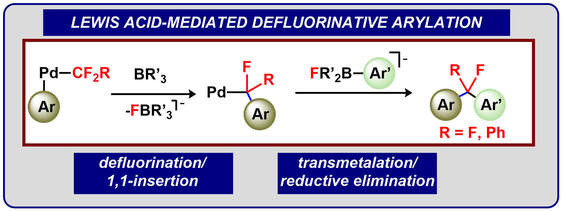
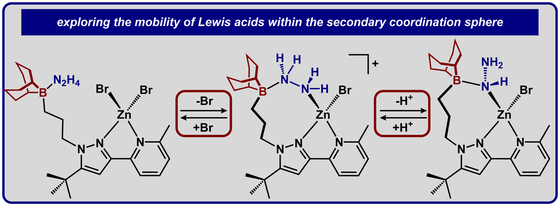
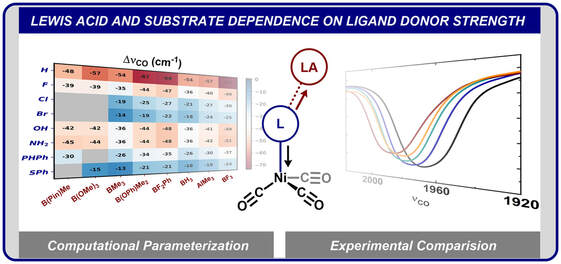
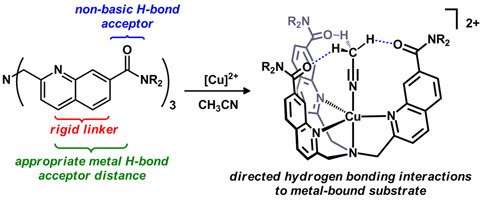
42.) Shanahan, J. P.; Szymczak, N. K..; Lewis Acid Effects on Calculated Ligand Electronic Parameters
Organometallics. 2020, 39, 23, 4297-4306.
***Special issue: Organometallic Chemistry of the Main-Group Elements
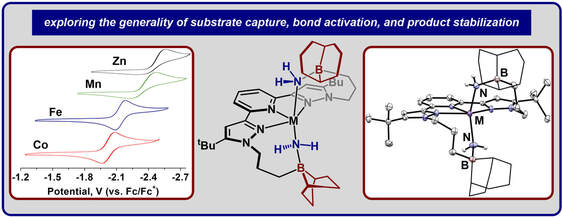
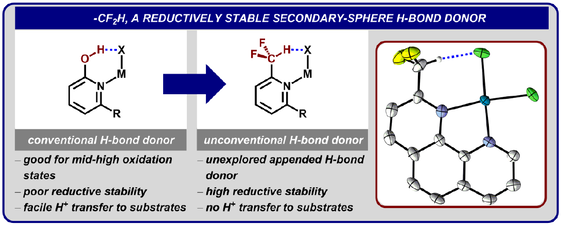
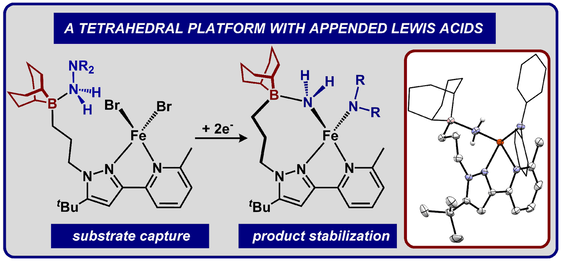
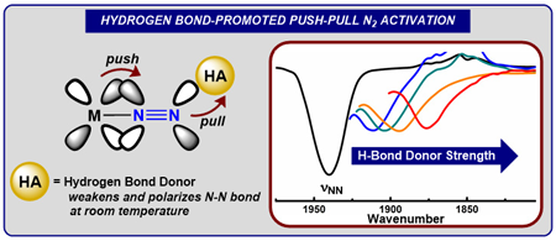
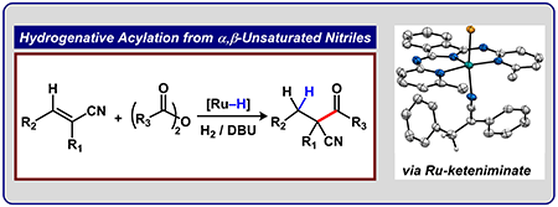
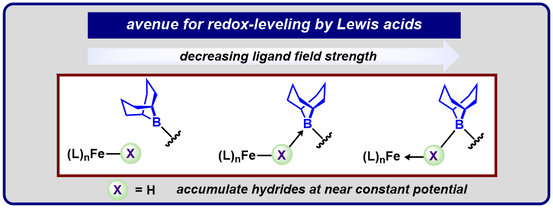
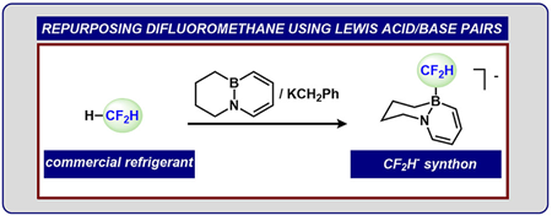
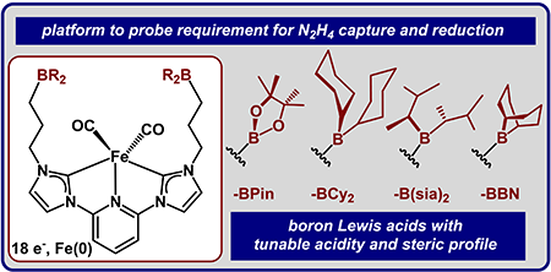
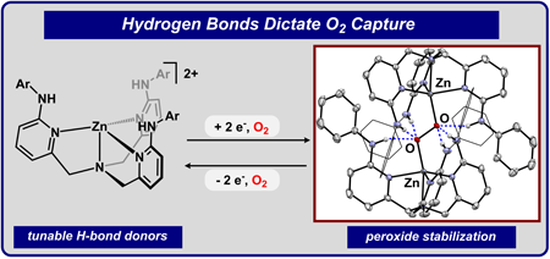
Graduate and Postdoctoral Publications
17.) McCrory, C. C. L.; Szymczak, N. K.; Peters, J. C.; Evaluating Activity for Hydrogen-Evolving Cobalt and Nickel Complexes at Elevated Pressures of Hydrogen and Carbon Monoxide.
Electrocatalysis 2016, 7, 87-96
16.) Bayram, E.; Linehan, J. C.; Fulton, J. L.; Szymczak, N. K.; Finke, R. G.; Determination of the Dominant Catalyst Derived from the Classic [RhCp*Cl2]2 Precatalyst System: Is it Single-Metal Rh1Cp*-Based, Subnanometer Rh4 Cluster-Based, or Rh(0)nNanoparticle-Based Cyclohexene Hydrogenation Catalysis at Room Temperature and Mild Pressures?
ACS Catal. 2015, 5, 3876-3886.
15.) Ercan, B.; Linehan, J.; Fulton, J.; Roberts, J.; Szymczak, N.; Smurthwaite, T.; Ozkar, S.; Balasubramanian, M.; Finke, R. Is It Homogeneous or Heterogeneous Catalysis Derivedfrom [RhCp*Cl2]2? In Operando-XAFS, Kinetic and Crucial Kinetic Poisoning Evidence for Subnanometer Rh4 Cluster-Based Benzene Hydrogenation Catalysis.
J. Am. Chem. Soc. 2011, 133, 18889-18902.
14.) Neiner, D.; Karkamamkar, A.; Bowden, M.; Choi, Y. J.; Luedtke, A.; Holladay, J.; Fisher, A.; Szymczak, N.; Autrey, T. Kinetic and Thermodynamic Investigation of Hydrogen Release from Ethane 1,2-Di-Amineborane.
Energy Environ. Sci. 2011, 4, 4187-4193.
13.) Szymczak, N. K.; Berben, L. A.; Peters, J. C. Redox-Rich Dicobalt Macrocycles as Templates for Multi-Electron Transformations.
Chem. Commun. 2009, 6729-6731.
12.) Szymczak, N. K.; Braden, D. A.; Crossland, J. L.; Turov, Y.; Zakharov, L. N.; Tyler, D. R. Aqueous Coordination Chemistry of H2. Why is Coordinated H2 Inert to Substitution by Water in trans-Ru(P2)2(H2)H+-type Complexes (P2 = a Chelating Phosphine)?
Inorg. Chem. 2009, 48, 2976-2984.
11.) Yelle, R. B.; Crossland, J. C.; Szymczak, N, K.; Tyler, D. R. Theoretical Studies of N2 Reduction to Ammonia in Fe(dmpe)2N2.
Inorg. Chem. 2009, 48, 861-871.
10.) Pons, V; Baker, R. T.; Szymczak, N. K.; Heldebrant, D. J.; Linehan, J. C.; Matus, M. H.; Grant, D. J.; Dixon, D. A. Coordination of Aminoborane, NH2BH2, Dictates Selectivity and Extent of H2 Release in Metal-Catalysed Ammonia Borane Dehydrogenation.
Chem. Commum. 2008, 48, 6597-599.
9.) Shaw, W. J; Linehan, J. C.; Szymczak, N. K.; Heldebrant, D. J.; Yonker, C.; Baker, R. T.; Autrey, T. In Situ Multinuclear NMR Spectroscopic Studies of the Thermal Decomposition of Ammonia Borane in Solution.
Angew. Ch., Int. Ed. 2008, 120, 7603-7606.
8.) Szymczak, N. K.; Tyler, D. R. Aspects of Dihydrogen Coordination Chemistry Relevant to Reactivity in Aqueous Solution.
Coord. Chem. Rev. 2008, 252(1-2), 212-230.
7.) Fulton, J. L.; Linehan, J. C.; Autrey, T.; Balasubramanian, M.; T.;Chen, Y.; Szymczak, N. K.. When is a Nanoparticle a Cluster? An Operando EXAFS Study of Amine Borane Dehydrocoupling by Rh4-6 Clusters.
J. Am. Chem. Soc. 2007, 129, 11936-11949.
6.) Gilbertson, J. D.; Szymczak, N, K.; Crossland, J. C.; Miller, W. K.; Lyon, D. K.; Foxman, B. M.; Davis, J.; Tyler, D. R. Water-Soluble Transition Metal Phosphine Complexes: Investigation of the Aqueous Binding and Activation of H2 and N2 in trans-FeII(P2)2X2-type Complexes (P2 = a Chelating Phosphine).
Inorg. Chem. 2007, 46, 1205-1214.
5.) Szymczak, N. K.; Zakharov, L. N.; Tyler, D. R. Solution Chemistry of a Water-Soluble n2-H2 Complex: Evidence for H2 acting as a Hydrogen Bond Donor.
J. Am. Chem. Soc. 2006, 128, 15830-15835.
4.) Szymczak, N. K.; Oelkers, A. B.; Tyler, D. R. Detection of Hydrogen Bonding in Solution: A 2H Nuclear Magnetic Resonance Method Based on Rotational Motion of a Donor/Acceptor Complex.
Phys. Chem. Chem. Phys. 2006, 8, 4002-4008.
3.) Gilbertson, J. D.; Szymczak, N. K.; Tyler, D. R. Reduction of N2 to Ammonia and Hydrazine Utilizing H2 as the Reductant.
J. Am. Chem. Soc. 2005, 127, 10184-10185.
2.) Szymczak, N. K.; Han, F.; Tyler, D. R. Arrested Chloride Abstraction from trans-RuCl2(DMeOPrPE)2 with TlPF6; Formation of a 1-D Coordination Polymer having Unusual Octahedral Coordination around Thallium(I).
J. Chem. Soc., Dalton Trans. 2004, 3941-3942.
1.) Gilbertson, J. D.; Szymczak, N. K.; Tyler, D. R. H2 Activation in Aqueous Solution: Formation of trans-[Fe(DMeOPrPE)2H(H2)]+via the Heterolysis of H2 in Water.
Inorg. Chem. 2004, 43, 3341-3343.
17.) McCrory, C. C. L.; Szymczak, N. K.; Peters, J. C.; Evaluating Activity for Hydrogen-Evolving Cobalt and Nickel Complexes at Elevated Pressures of Hydrogen and Carbon Monoxide.
Electrocatalysis 2016, 7, 87-96
16.) Bayram, E.; Linehan, J. C.; Fulton, J. L.; Szymczak, N. K.; Finke, R. G.; Determination of the Dominant Catalyst Derived from the Classic [RhCp*Cl2]2 Precatalyst System: Is it Single-Metal Rh1Cp*-Based, Subnanometer Rh4 Cluster-Based, or Rh(0)nNanoparticle-Based Cyclohexene Hydrogenation Catalysis at Room Temperature and Mild Pressures?
ACS Catal. 2015, 5, 3876-3886.
15.) Ercan, B.; Linehan, J.; Fulton, J.; Roberts, J.; Szymczak, N.; Smurthwaite, T.; Ozkar, S.; Balasubramanian, M.; Finke, R. Is It Homogeneous or Heterogeneous Catalysis Derivedfrom [RhCp*Cl2]2? In Operando-XAFS, Kinetic and Crucial Kinetic Poisoning Evidence for Subnanometer Rh4 Cluster-Based Benzene Hydrogenation Catalysis.
J. Am. Chem. Soc. 2011, 133, 18889-18902.
14.) Neiner, D.; Karkamamkar, A.; Bowden, M.; Choi, Y. J.; Luedtke, A.; Holladay, J.; Fisher, A.; Szymczak, N.; Autrey, T. Kinetic and Thermodynamic Investigation of Hydrogen Release from Ethane 1,2-Di-Amineborane.
Energy Environ. Sci. 2011, 4, 4187-4193.
13.) Szymczak, N. K.; Berben, L. A.; Peters, J. C. Redox-Rich Dicobalt Macrocycles as Templates for Multi-Electron Transformations.
Chem. Commun. 2009, 6729-6731.
12.) Szymczak, N. K.; Braden, D. A.; Crossland, J. L.; Turov, Y.; Zakharov, L. N.; Tyler, D. R. Aqueous Coordination Chemistry of H2. Why is Coordinated H2 Inert to Substitution by Water in trans-Ru(P2)2(H2)H+-type Complexes (P2 = a Chelating Phosphine)?
Inorg. Chem. 2009, 48, 2976-2984.
11.) Yelle, R. B.; Crossland, J. C.; Szymczak, N, K.; Tyler, D. R. Theoretical Studies of N2 Reduction to Ammonia in Fe(dmpe)2N2.
Inorg. Chem. 2009, 48, 861-871.
10.) Pons, V; Baker, R. T.; Szymczak, N. K.; Heldebrant, D. J.; Linehan, J. C.; Matus, M. H.; Grant, D. J.; Dixon, D. A. Coordination of Aminoborane, NH2BH2, Dictates Selectivity and Extent of H2 Release in Metal-Catalysed Ammonia Borane Dehydrogenation.
Chem. Commum. 2008, 48, 6597-599.
9.) Shaw, W. J; Linehan, J. C.; Szymczak, N. K.; Heldebrant, D. J.; Yonker, C.; Baker, R. T.; Autrey, T. In Situ Multinuclear NMR Spectroscopic Studies of the Thermal Decomposition of Ammonia Borane in Solution.
Angew. Ch., Int. Ed. 2008, 120, 7603-7606.
8.) Szymczak, N. K.; Tyler, D. R. Aspects of Dihydrogen Coordination Chemistry Relevant to Reactivity in Aqueous Solution.
Coord. Chem. Rev. 2008, 252(1-2), 212-230.
7.) Fulton, J. L.; Linehan, J. C.; Autrey, T.; Balasubramanian, M.; T.;Chen, Y.; Szymczak, N. K.. When is a Nanoparticle a Cluster? An Operando EXAFS Study of Amine Borane Dehydrocoupling by Rh4-6 Clusters.
J. Am. Chem. Soc. 2007, 129, 11936-11949.
6.) Gilbertson, J. D.; Szymczak, N, K.; Crossland, J. C.; Miller, W. K.; Lyon, D. K.; Foxman, B. M.; Davis, J.; Tyler, D. R. Water-Soluble Transition Metal Phosphine Complexes: Investigation of the Aqueous Binding and Activation of H2 and N2 in trans-FeII(P2)2X2-type Complexes (P2 = a Chelating Phosphine).
Inorg. Chem. 2007, 46, 1205-1214.
5.) Szymczak, N. K.; Zakharov, L. N.; Tyler, D. R. Solution Chemistry of a Water-Soluble n2-H2 Complex: Evidence for H2 acting as a Hydrogen Bond Donor.
J. Am. Chem. Soc. 2006, 128, 15830-15835.
4.) Szymczak, N. K.; Oelkers, A. B.; Tyler, D. R. Detection of Hydrogen Bonding in Solution: A 2H Nuclear Magnetic Resonance Method Based on Rotational Motion of a Donor/Acceptor Complex.
Phys. Chem. Chem. Phys. 2006, 8, 4002-4008.
3.) Gilbertson, J. D.; Szymczak, N. K.; Tyler, D. R. Reduction of N2 to Ammonia and Hydrazine Utilizing H2 as the Reductant.
J. Am. Chem. Soc. 2005, 127, 10184-10185.
2.) Szymczak, N. K.; Han, F.; Tyler, D. R. Arrested Chloride Abstraction from trans-RuCl2(DMeOPrPE)2 with TlPF6; Formation of a 1-D Coordination Polymer having Unusual Octahedral Coordination around Thallium(I).
J. Chem. Soc., Dalton Trans. 2004, 3941-3942.
1.) Gilbertson, J. D.; Szymczak, N. K.; Tyler, D. R. H2 Activation in Aqueous Solution: Formation of trans-[Fe(DMeOPrPE)2H(H2)]+via the Heterolysis of H2 in Water.
Inorg. Chem. 2004, 43, 3341-3343.